1999
Did the research and development of the blood lipid-lowering agent intermediate "Compactin" in the open laboratory of the Food Industry Development Institute.
Obtained the SBIR subsidy from the Ministry of Economic Affairs "Production Technology Innovation Project of Compactin, an Intermediate of Hypolipidemic Agent".

The R&D results surpassed the target yield of the SBIR project.
Built a new plant and set up two sets of 5,000L fermentation equipment at Sinying for R&D and mass production of API intermediates.

2001
Set up Taipei Sales Department to expand domestic and foreign markets.
Massively produced edible and medicinal mushrooms and functional fermented products.
Shortlisted for the First Science and Technology Star Award by the Ministry of Economic Affairs.

2003
Speedily obtained the approval of the National Science Council Review Committee to enter the Tainan Science Park.
Mapped out to build a high-tech factory in Tainan Science Park that complies with cGMP standards.

The plant 1 in Tainan Science Park was completed and successfully activated 50,000L fermentation mass production equipment.
Obtained ISO 9001 : 2000 International Quality Certificate

2005
Awarded as Excellent Manufacturer by the Minister of Labor Republic of China (Taiwan).
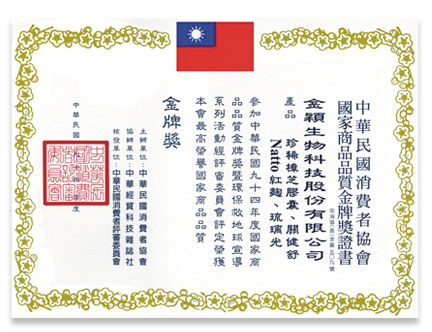
Obtained the "National Product Quality Gold Medal".
Obtained ISO 22000 and HACCP International Quality Certificates.
Co-published the research results of "Phellinus linteus: Anti-liver Fibrosis" with College of Medicine, National Cheng Kung University.


2008
Signed a contract with Kemin to become its Asian mass production and delivery center.
Cooperated with the Medical Center to verify the benefits of oral nattokinase products for patients with dyslipidemia.

Obtained the Halal certification of Islamic food, actively expand the Muslim market at home and abroad.

2011
Established Shanghai branch.
Cooperated with the Medical Center to verify the effectiveness of NattoMena®, natural vitamin K2, on preventing osteoporosis and vascular calcification.

Acquired the patent of Antrodia cinnamomea mycelium liquid capsule.
Published the fingerprint and pharmacokinetic research of Antrodia cinnamomea studies in international journals.
NattoMena® gained SNQ National Quality award.



2013
Signed a tripartite cooperation with a German biotechnology company and Food Industry Research and Development Institute to develop and mass produce key enzymes.
Cooperate with a Japanese hundred-year factory to conduct development and mass production of fermentation products.
2015
Participated in Guan-Rong program
Established Dong Guan branch
Expanded with two sets of 55,000L fermentation equipment

2017
Expanded with three sets of drying equipment at the plant 1 in Tainan Science Park
Built a filling and packaging plant that meets GHP specifications at Sinying factory
Obtained Dun & Bradstreet Elite SME Award
Awarded the honor of "Deloitte Asia-Pacific High-tech, High-growth top 500"

Officially listed on TPEx-Listed Stocks on January 22, 2018 (Stock Code: 1796)
Obtained NSF‐DS GMP Certificate, which is recognized by the American National Standards Institute (ANSI) and the Standard Council of Canada (SCC).
NattoMena® obtained Gold Award at International Innovation & Invention Competition

Highly active nattokinase obtained the Japan patent
37Labtico® obtained China patent
LaLaBling™ enzyme obtained Gold Medal at IIIC
GSH12X™、37Labtico® obtained three gold medals at 2020 Tokyo Invention Exhibition (GSH12X™, Dual-efficacy Polysaccharides) and 2020 Korea WiC (37Labtico®)
LaLaBling™ enzyme obtained Gold Medal at IIIC
GeneFerm was awarded as TOP 5000 Outstanding Enterprise in 2020.



GeneFerm’s “Ganoderma lucidum polysaccharides composite composition” has obtained United States and Taiwanese invention patents.
GeneFerm cooperate with National Cheng Kung University for lean manufacturing application, and was supported by TPS program subsidies from the Ministry of Economic Affairs (Taiwan).
The new intelligent manufacturing Plant 2 at Southern Taiwan Science Park was completed, together with three 66,000-liter fermentation and extraction tanks, mass production equipment such as specialized centrifuge, freeze drying and large-scale spray drying facilities.

2023
GeneFerm has been assessed and determined to comply with the requirements of Standard FOOD SAFETY SYSTEM CERTIFICATION 22000 FSSC 22000